Original Source: http://www.natap.org/
“I think the information suggests that this test could be very significant in helping to stem the number of new cases of HIV that occur every year,” said Blaine Hollinger, who chairs the panel. Some critics of the test have raised questions about its accuracy, but the benefits outweigh the harm of the occasional misdiagnosis, the panel said……The test, which looks for signs of HIV in oral fluid, is already used at hospitals and doctors’ offices where medical professionals administer it. The FDA first approved its use in 2004.
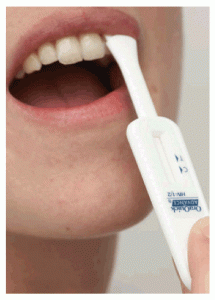
healthday: The test, which looks for signs of HIV in oral fluid, is already used at hospitals and doctors’ offices where medical professionals administer it. The FDA first approved its use in 2004. To take the test, known as OraQuick, patients swab their outer gums and put the swab into a vial. After about 20 minutes, the test device will reveal two reddish-purple lines in a small window if there are signs that the body’s immune system has geared up to battle HIV.
The test uses oral fluid, which is not the same as saliva. Its results are considered preliminary, and should be confirmed by a blood test.To take the test, known as OraQuick, patients swab their outer gums and put the swab into a vial. After about 20 minutes, the test device will reveal two reddish-purple lines in a small window if there are signs that the body’s immune system has geared up to battle HIV.
Pant Pai said the oral test’s overall accuracy is similar to that of a blood test, although it’s slightly less accurate. The oral test, in particular, may miss HIV infection in its early stages, she said.
Also, “the sensitivity of the test appears lower when administered in the home setting rather than a medical setting, so some of the people who are HIV-positive will get a test result that they are negative,” said Jane Rotheram-Borus, director of the Center for HIV Identification Prevention and Treatment Services at the University of California, Los Angeles. “However, if they would otherwise not have gotten the test at all, they may also have believed they were negative.”
In addition to talking about its accuracy, the FDA panel may also examine the support that’s available for people who learn at home, possibly alone, that they are probably infected with the virus that causes AIDS.
“The arguments against the at-home test focus on the absence of a counselor who could provide support and link the newly identified HIV-positive individual to medical care,” said Rotheram-Borus, who supports over-the-counter sales of the OraQuick test.
She pointed out that “over-the-counter pregnancy tests are widely used, and pregnant women do find their way into prenatal care.” (from Jules: one person testified in public open mic that pregnancy tests are just as insensitive or inaccurate).
The company that makes the test says it will offer a 24-hour toll-free number that people can call to get support regarding their test results.
WSJ: It isn’t clear if the home test provided a negative result or if people read the test incorrectly. One measure of accuracy of the OraQuick home test is 93%, but when used by health-care professionals, that level is 99.3%.
There may be other limits to the test. Julie Scofield, executive director of the National Alliance of State and Territorial AIDS Directors, a group that represents state health departments, said in a letter to the FDA that consumers may not be able to detect infections acquired in the previous four to six weeks before testing.
Still, FDA officials said the home-use test would likely encourage more people to get tested. “We believe there would be public-health benefits” of a home-based HIV test, said Richard Forshee, an FDA associate director for research. “But an individual risk remains in the form of an increase in the number of false negatives.”The test uses a swab to collect salvia inside a person’s mouth. After the swab is inserted into a test tube for 20 minutes, the test indicates whether a person is infected with HIV. The company and the FDA said positive tests should be confirmed with a blood test.
MSNBC: U.S. health officials told the panel that home-testing could help get needed healthcare to HIV-positive individuals earlier. At present, only 62 percent of those with HIV are linked to the healthcare services and just 28 percent have access to drugs capable of suppressing the infection.
CNN Blog
Consumers may soon be able to test themselves for HIV and quickly learn the results in the privacy of their own homes following a unanimous approval recommendation from a Food and Drug Administration advisory committee on Tuesday.
The panel said the OraQuick In-Home HIV Test should be made available over-the-counter (OTC) saying the test is safe and effective and that the benefits far outweigh the potential risks.
If approved by the FDA, the test will be the first OTC test to be marketed for HIV or any infectious disease. FDA advisory committee recommendations are not binding, but they are generally followed.
An estimated 1.2 million Americans are living with HIV, according to the Centers For Disease Control and Prevention. One in five of those are unaware of their HIV status. And about 50,000 new cases of HIV are reported each year.
OraSure Technologies, Inc., the manufacturer of this new test, also makes the already approved OraQuick ADVANCE Rapid HIV-1/2 Antibody Test.
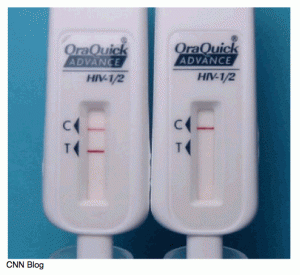
That test can only be used in a clinical setting and results are provided in 20 minutes. The In-home test is a modified version where the individual swabs the upper and lower gums with a test pad device. That device is then inserted into a vial of solution. Much like a pregnancy test, one line shows up if the test is negative, two lines means it’s positive.
The kit includes step-by-step instructions on when to test, how to set-up and administer the test, and how to interpret the results. The company also provides information on how to follow-up on the results through OraQuick’s Answer Center for support and local medical referral is also provided. The center is staffed 24 hours a day, 7 days a week.
“The test is designed to be comprehensive across a broad range of literacy levels,” said Stephen Lee, OraSure’s executive vice president and chief science officer. “It enables use in a variety of testing environments.”
According to Lee the test has a high degree of accuracy and 93% sensitivity. In clinical studies the test proved 99.98% effective in determining who did not have the virus. Panelists were concerned about making sure there was comprehensive language in the kit regarding a testing window. Specifically, you must wait 3 months between high risk behaviors before testing. Another major concern was the risk of false positives and false negatives.
But for the 25 or so health care professional and HIV/AIDS advocates that addressed the panel, imploring the committee to vote for approval, those risks did not outweigh the opportunity to expand public access to testing and reduce the number of infections by making sure people know their HIV status. That a test could be done in the privacy of their own homes was seen as an added bonus – and a way to address the stigma surrounding HIV. Nobody requested that the test should not be approved.
“We need to do whatever we need to move the needle of the number of people who don’t know their status,” said Whitney Cordova of the AIDS Healthcare Foundation. “This test helps do that. The perfect cannot be the enemy of the good.”
“It is clear OTC is not the right way to be tested for everyone,” said Tom Donohue, Jr., founder of Who’s Positive. “OTC is another tool. OTC has the potential to reach a far greater number of individuals who want to know their HIV status on their own terms. It’s time for everyone to know their HIV status. It’s time to give people tools without barriers to know their status.”
If the OraQuick In-Home HIV Test gets final FDA approval, hopes are high that this new tool will be instrumental in helping to reduce infection rates in the U.S. and globally.
There are “1.2 million people living with AIDS in the U.S. – and 20% don’t know it. This is a ticking time bomb,” said Frank Oldham, president and CEO of the National Association of People With AIDS. “We need more weapons to reduce HIV infections and we are morally obligated to bring this test to market and reduce the spread HIV.”
Cornelius Baker, technical adviser for the FHI 360 Center on AIDS & Community Health, underscored the value of testing at home with rapid results.
“This is especially important for those who will not seek testing in a public place or who need the convenience because of geography or time to test more frequently with this new method,” Baker said. “If we are to win the battle against the HIV epidemic, we need to employ multiple strategies. While [this is] not a product that every person will use or need, this in-home test is one more contribution to helping every American become part of ending AIDS in the United States.”
FDA presenters clearly felt the test had merit.
“Greater access to testing will potentially lead to more people knowing their HIV status.” said Dr. Elliot Cowan, who spoke on behalf of the FDA. “It appears there is a benefit in increasing the number of HIV positive people who know their status. There is public health impact and personal health impact.”
OraSure Executives seemed confident of approval calling it a momentous day for public health. “We plan to work with the FDA over the coming months to refine product label enhancements,” said Douglas Michaels, the company’s president and CEO. “And as quickly as we can get that done will determine how quickly we can get the product to retailers.”
Michaels says they have not yet finalized their pricing, but will sell the test to retailers at a discount and ultimately retailers will set the price.
———————–
US drug panel “backs over-the-counter HIV tests”
Wednesday, May 16, 2012,15:26
The Food and Drug Administration regulators will decide later this year whether to approve it; it does not have to follow the recommendations of the advisory panel, though it usually does, the `BBC` reported.
The 20-minute test is 93 per cent accurate for positive results and 99.8 per cent for negative, the manufacturer said.
To take the test, the user swabs the outer gum area so the oral fluid, which is not the same as saliva, can be checked for the HIV virus. The test provides results within about 20 minutes, but the experts say the results should be confirmed with a blood test, which is more accurate.
In fact, the experts on the Blood Products Advisory Committee voted 17-0 to back the test, saying it would help people who are HIV-positive get access to healthcare and social services.
The experts urged Pennsylvania-based OraSure, the company that manufactures the product, to include highly visible warnings about false negative results. The panel also advised that the packaging should carry a toll-free phone number offering counselling to those testing positive.
OraSure said the home test could retail for less than US dollars 60 if approved.
Carl Schmid, deputy director of the AIDS Institute, has welcomed the panel`s approval on Tuesday of the home test.
“We are always looking for game changers, and we believe this is one of them. Not only will it help reduce the number of infections but it will bring more people into care and treatment,” he was quoted as saying.
————————-
FDA panel backs first rapid, take home HIV test
Businessweek, Washington The Associated Press May 15, 2012, 6:29PM ET By MATTHEW PERRONE
American consumers may soon be able to test themselves for the virus that causes AIDS in the privacy of their own homes, after a panel of experts on Tuesday recommended approval of the first rapid, over-the-counter HIV test.
The 17 members of the Food and Drug Administration advisory panel voted unanimously that the benefits of the OraQuick HIV test outweigh its potential risks for consumers. While the test, which uses a mouth swab to return a result in 20 minutes, does not appear to be as accurate as professionally-administered diagnostics, panelists said it could provide an important way to expand HIV testing.
The FDA will make its final decision on whether to approve the product later this year, weighing the opinion of the panel.
Government officials estimate one-fifth, or about 240,000 people, of the 1.2 million HIV carriers in the U.S. are not aware they are infected. Testing is one of the chief means of slowing new infections, which have held steady at about 50,000 per year for two decades.
Based in Bethlehem, Pa., Orasure has marketed a version of OraQuick to doctors, nurses and other health care practitioners since 2004. When used by professionals, the test is shown to accurately identify both carriers and non-carriers 99 percent of the time.
But a trial conducted by the company showed the home test only correctly detected HIV in those carrying the virus 93 percent of the time. The FDA estimated the test would miss about 3,800 HIV-positive people per year, while correctly identifying 45,000, if approved for U.S. consumers. The test could prevent 4,000 new transmissions of the virus annually, though the figure could vary depending on how many people purchase the test. While it’s not clear why the test was less accurate in consumer trials, company researchers said they expected the test’s sensitivity to drop when used by consumers versus professionals.
Panelists stressed that the test’s labeling should state that a negative reading does not automatically mean the person does not have HIV, because of imperfect results. Panelists also stressed the importance of a toll-free number to put those who test positive in touch with counseling and medical care.
Despite concerns about less-than-perfect use by the public, the panel overwhelmingly sided with more than two dozen HIV advocates and doctors, who said the test represents a step forward in combating the virus.
“Over-the-counter testing has the potential to reach a far greater number of people who want to know their HIV status on their own terms,” said Tom Donohue, founder of HIV awareness group Who’s Positive.
“We are always looking for game changers, and we believe this is one of them,” said Carl Schmid, deputy director of the AIDS Institute. “Not only will it help reduce the number of infections but it will bring more people into care and treatment.”
Tuesday’s meeting was the second time in less than a week that FDA advisers recommended approval of a novel medical product to slow the spread of HIV. Last Thursday a similar panel of drug experts endorsed the HIV daily pill Truvada for preventive use. If FDA follows the group’s advice, the daily medication will become the first drug approved to prevent healthy people from becoming infected with the virus.
The FDA has already approved other HIV test kits designed to be used at home, although those kits — which require a blood sample — must be sent to a laboratory for development. Orasure executives argue that a test that can be done at home will appeal to a much broader group of people.
FDA officials said it is impossible to predict how widely the test would be used, but it would be most effective in the hands of people at high risk of acquiring the virus, including men who have sex with men.
A recent survey of gay and bisexual men cited by public health officials found that 84 percent would test themselves more frequently if they could buy an over-the-counter HIV test.
Orasure sells its professional version of the test for $17.50, though company executives declined to discuss how they would price the consumer version.
Home HIV test advances
An FDA advisory panel approved the oral swab screening.
By Don Sapatkin
Inquirer Staff Writer
| OraQuick’s test gives results in about 20 minutes. About 240,000 Americans do not know they are HIV positive.A federal advisory committee on Tuesday unanimously approved over-the-counter sale of a rapid HIV test, acknowledging the need for new tools against an epidemic that is driven largely by people who don’t know their status and infect others.If the Food and Drug Administration agrees with its advisers, the oral swab screening device made by OraSure Technologies Inc. of Bethlehem, Pa., would become the first infectious disease test approved for home use.The panel overcame considerable unknowns and concerns that the test cannot pick up newly acquired infections.”I still can’t get past the quarter-million people who don’t get tested,” said member Steven W. Pipe, a pediatrics and communicable diseases specialist at the University of Michigan.An estimated 240,000 Americans are unaware that they are HIV positive, and are responsible for 50 percent to 70 percent of the 50,000 new infections each year.The test uses fluid from a swab of the gums. If a single horizontal line appears after 20 minutes, it has not detected antibodies to HIV. Two lines mean it is HIV positive, a preliminary finding that must be confirmed by a laboratory blood test.”We believe we are at a deciding moment in the trajectory of the AIDS epidemic,” Phill Wilson, executive director of the Black AIDS Institute, told the panel, referring to treatments that dramatically reduce transmission of HIV, the virus that causes AIDS. “The key to that begins with getting them tested.”The company has not disclosed a retail price or projected sales, although it estimated the entire market for rapid home HIV tests at $500 million retail. FDA staff projected that the test would be used by 2.8 million people in the first year. It would discover about 45,000 infections that otherwise would have remained unknown, and in turn, prevent HIV from spreading to 4,000 more people, the agency said.But it also would result in several thousand false negatives, either because consumers didn’t use it properly or their infections were too new to trigger the immune system response that produces antibodies picked up by the test.
Much of Tuesday’s discussion among the advisory committee was about labeling and other ways to make clear to people at high risk of HIV that a negative test should be repeated three months later or confirmed with a laboratory blood test. Of particular concern was that people not use a negative result as a free pass to engage in risky sex.
The company has said that each test kit would include two booklets – one with detailed information about HIV and the other on the meaning of test results – in English and Spanish. It also would refer people to a 24-hour call center. In addition to helping people understand their test results, the bilingual staff would be trained as crisis counselors and could link callers to local counseling and a medical treatment.
In a teleconference with reporters after the advisory panel’s 17-0 vote, OraSure officials said they would immediately begin working with the FDA to address labeling issues.
“We are extremely pleased with the outcome of today’s meeting,” said Douglas A. Michels, president and CEO of the company, which has 270 employees.
The company has sold more than 25 million rapid HIV tests to clinics and testing centers around the world since 2002, when it won approval for the first rapid blood test for HIV. All were manufactured in Bethlehem.
The product that was recommended for home use on Tuesday is identical to the oral version of that test, which was approved in 2004, except for the packaging, labeling, and support services.
As a result, the company has said it could begin selling the test at pharmacies and online within 60 days of approval by the FDA. The agency typically follows its advisers’ recommendations but is not required to.
More than 20 HIV activists, doctors, and public health workers testified at the Blood Products Advisory Committee hearing. They overwhelmingly supported the OraQuick In-Home HIV Test, often in passionate terms.
“I envision my parishioners coming to me and saying, ‘Pastor, will you sit 20 minutes with me while I take the test?’ ” said Calvin O. Butts 3d, of the Abyssinian Baptist Church in New York, adding that they might never go to a clinic for a test and so would never know whether they had HIV.
Tom Donohue, who grew up in Williamsport, Pa., recalled discovering he was HIV positive while a student at Penn State University.
“I know what it’s like to wait for results, I know what it’s like to share my sexual history before a community member who is an HIV tester,” said Donohue, who founded and is executive director of Who’s Positive, an HIV awareness organization.
|