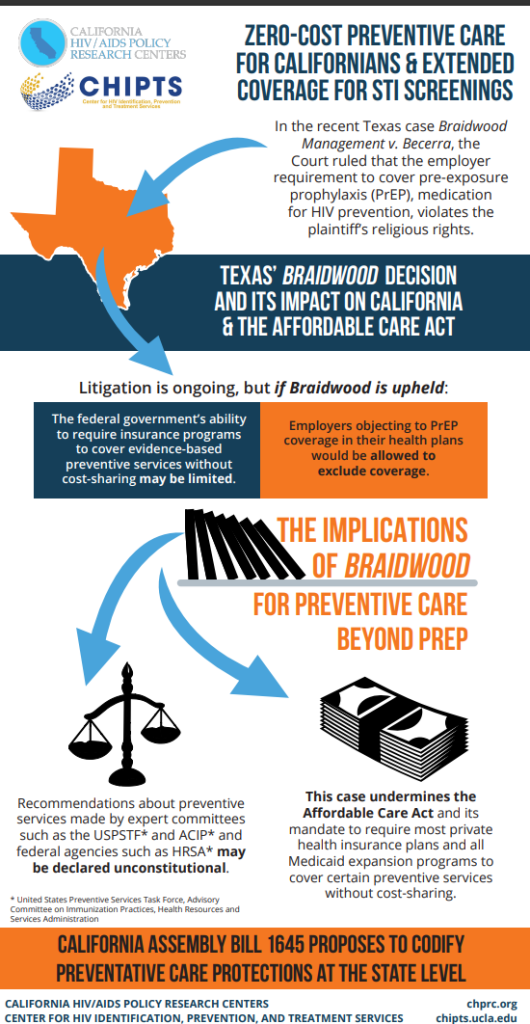
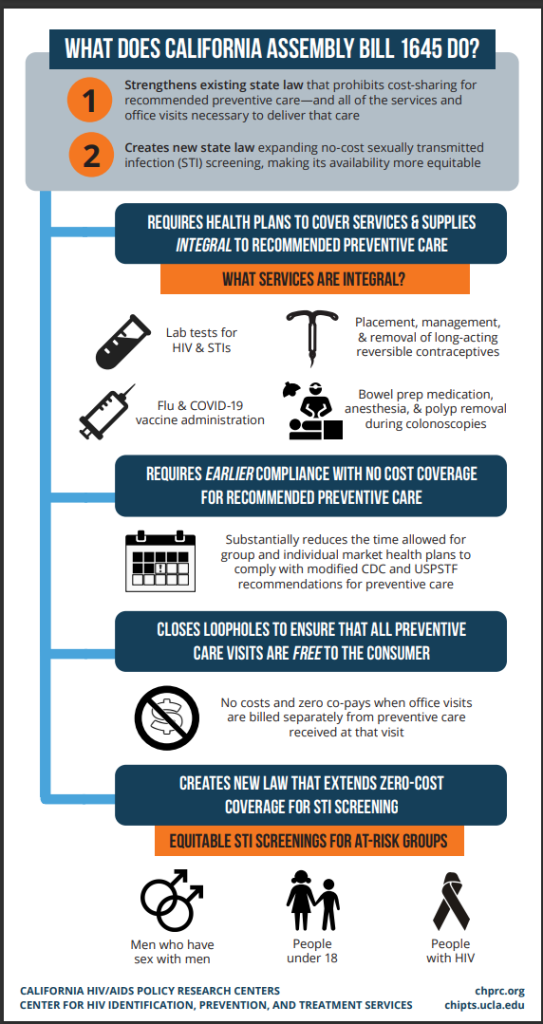
This content originally appeared on HIV.Gov. View the full article here.
Reflections on the IAS 2023 conference
When I joined NIH OAR earlier this year, my appreciation grew for the profound and complex nature of scientific discoveries made possible by NIH HIV funding and the institutional and community partnerships that enable this research. The recent International AIDS Society Conference on HIV Science (IAS 2023) in Brisbane, Australia, underscored the importance of highlighting the tremendous scientific efforts of intramural and extramural NIH researchers who continue to drive us closer to ending the HIV pandemic.
This biennial conference showcases science and scientific discovery in the continual learning process that is fighting HIV. This year’s conference provided new insights that reminded us how far we’ve come but also how far we still need to go.
Chief among these insights were the findings from the REPRIEVE clinical trial, a large global study supported by the National Institute of Allergy and Infectious Disease (NIAID), the National Heart, Lung, and Blood Institute (NHLBI), and OAR. The study found that statins can reduce major cardiovascular events by more than a third in people with HIV. When investigators measured the combination of major cardiovascular events or premature deaths from any cause, use of a statin reduced this number by one in five in this population. Furthermore, this trial studied people with HIV who were classified as having low-to-moderate cardiovascular risk, a group that historically would not routinely be prescribed statins.
As a result, the findings are expected to directly influence clinical guidelines and standards of care for an aging population of people with HIV and add to the growing literature that demonstrates that people with HIV experience higher cardiovascular risk than the general population.
OAR has recognized the challenges faced by a growing population of people aging with HIV and established its HIV and Aging Signature Program. This program was developed in response to evolving community needs and feedback from federal and community partners. [At the] U.S. Conference on HIV and AIDS (USCHA) in Washington, DC, an NIH working group, co-led by OAR and the National Institute on Aging (NIA), [led] a panel discussion on key avenues of inquiry between HIV and aging that includes community members and representatives from relevant federal agencies.
Beyond the importance of REPRIEVE on cardiovascular outcomes, IAS 2023 also highlighted research into the long-term effects of certain classes of antiretroviral therapy on comorbidities, including hypertension, dyslipidemia, and weight gain, among people with HIV. The studies presented at the conference provide crucial insight for practitioners managing metabolic comorbidities of people with HIV, especially those older than 50.
Another topic of many sessions at IAS 2023 was the disproportionate impact of HIV on women and girls, who globally represent about half of all people with HIV and are the focus of OAR’s equity-informed and data-driven HIV and Women Signature Program. This program was launched in February 2023 in partnership with the NIH Office of Research on Women’s Health (ORWH).
The REPRIEVE study specifically examined outcomes among females with HIV, noting that while statins were equally effective across sexes, risk calculators may underestimate risk of cardiovascular disease among females with HIV, compared to males. In addition, females with HIV may have higher inflammatory markers despite lower prevalence of coronary artery plaque. These findings emphasize the importance of investigating sex-based differences to better understand the impact of comorbidities on women with HIV.
Findings from REPRIEVE and many other trials are helping close important research gaps. It is also critical to address existing science-to-service gaps, which OAR works to address through close collaboration and coordination with service-focused federal agencies.
Several studies presented at the conference aimed to narrow this science-to-service gap through behavioral, social, and implementation science to better understand how to reach a larger swath of the population of people with HIV or those who experience increased risk of HIV acquisition. Investigators presented data on preferences of adolescent girls and young women for using dapivirine vaginal rings over oral pre-exposure prophylaxis (PrEP). In addition, findings from HPTN 084-01, the long-acting injection for teens (LIFT) study, demonstrated the acceptability of long-acting injectables among adolescent females in Southern Africa.
These studies are critical to improve our understanding of which different treatment and prevention options are most effective for reaching different populations, allowing us to leverage our entire toolkit of interventions to end the HIV pandemic.
IAS 2023 put science front and center in the larger discussion about the HIV pandemic and served as an important reminder of its power to move the needle in our shared efforts to end the pandemic. If you would like to learn more about the findings discussed at IAS 2023 or about OAR’s Signature Programs, please watch this video interview with HIV.gov and review other insights shared at the conference.